Core-Shell Spherification® (CSS®)
Core-Shell Spherification® (CSS®) is Likarda’s proprietary platform technology for encapsulating cells, peptides, proteins, antibodies, and scaffolds in injectable hydrogel microbeads.
Unlike traditional encapsulation methods, CSS® combines your therapeutic with a liquid hydrogel that crosslinks into stable, precision-formed beads—tailored to your target site, release profile, and clinical need.
This flexible, modular system gives researchers and developers control over microbead size, degradation rate, and therapeutic loading—unlocking new delivery options for both cell-based and large/small molecule therapies. It can also turn slow-gelling biomaterials into injectable, targeted, long-acting delivery systems.
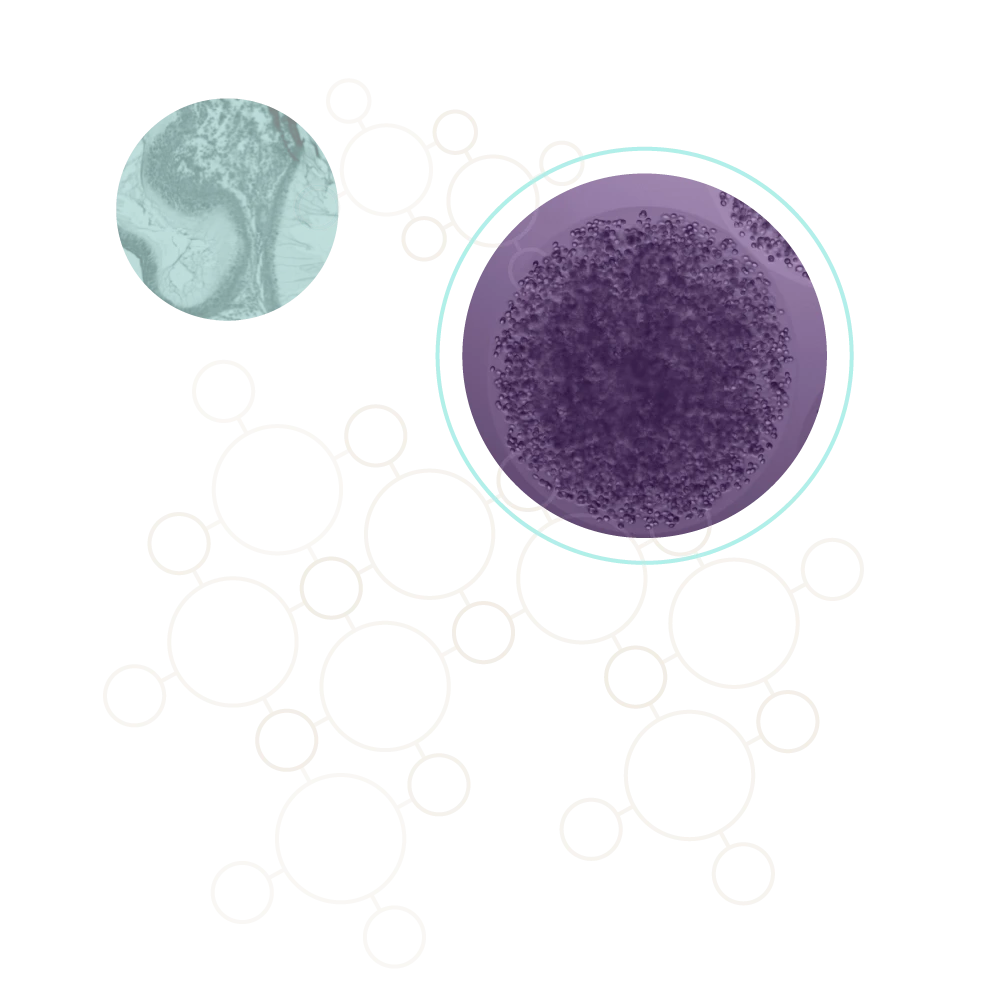
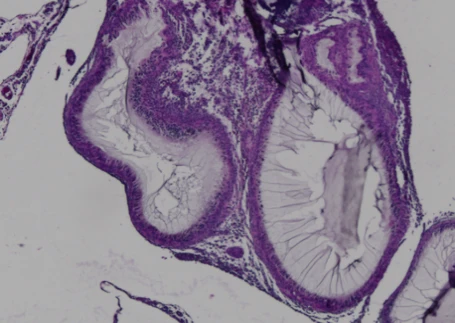
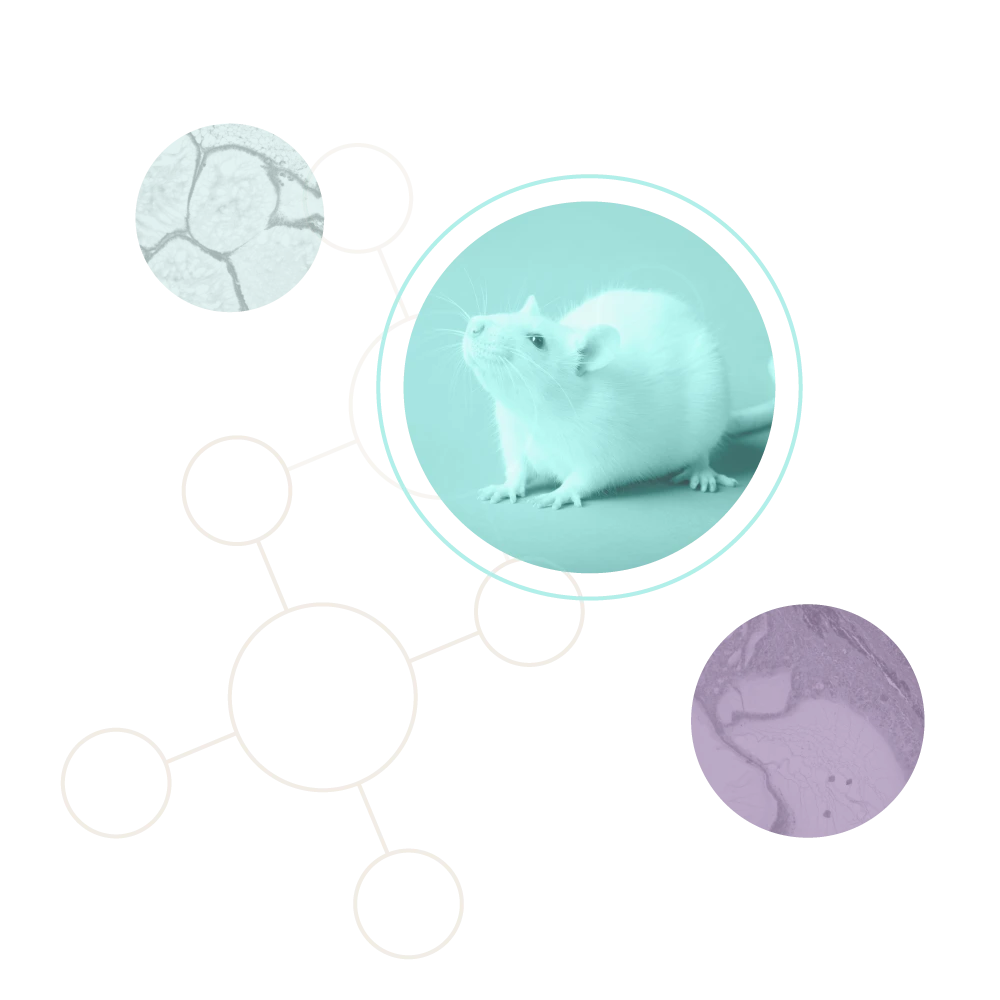
Encapsulated cells surrounded by fibrotic tissue, showing dense collagen deposition and loss of therapeutic viability due to the foreign body response.
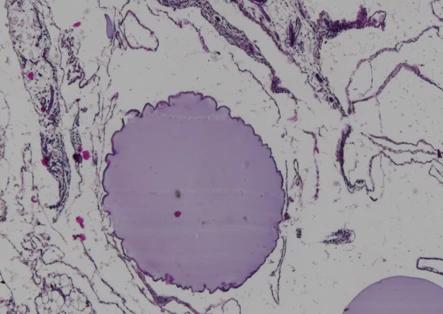
Cells encapsulated in CSS® hydrogel remain viable with minimal immune infiltration. The surrounding tissue shows healthy integration without fibrosis, demonstrating sustained protection and function
A Truly Customized Therapy Delivery System
Traditional delivery systems face major limitations—poor retention, immune rejection, burst release, cold-chain burden, and hard-to-scale manufacturing.
CSS® takes advantage of the well-established chemistry of hydrogels to create novel combinations of backbone chemistry and crosslinkers. The platform can generate microparticles in a variety of shapes, sizes, stiffness, and diffusion characteristics while maintaining complete control over the process.
The CSS® Promise:
Protect • Localize • Extend • Scale
- Protect: Immune-shielding, biocompatible hydrogels reduce rejection.
- Localize: Keep cells, proteins, or biologics at the site of action.
- Extend: Tunable release profiles from days to months.
- Scale: GMP-ready processes with a clear automation pathway.
How it works
Active ingredient (cell, biologic, peptide)
AI-guided recipes from proprietary data
Microencapsulation via our CSS instrument
Targeted injection: subQ, intratumoral, or oral (future)
Controlled, durable delivery
Why CSS® is Different
Customizable
Shell thickness, pore size, modulus, degradation
Compatible
Peptides, mAbs, fusion proteins, live cells
Durable release
Sustained profiles (days → months)
Biocompatibility focus
Materials & crosslinking optimized to minimize foreign-body response
Manufacturability
Closed-system processing; scale-out now, EncapSure™ automation (2026) for scale-up
Frequently Asked Questions
What hydrogel chemistries are supported?
CSS® is hydrogel-agnostic. We’ve demonstrated success with a broad range of biocompatible polymers including hyaluronic acid (HA), polyethylene glycol (PEG), polyvinyl alcohol (PVA), alginate alternatives, and proprietary blends. The platform allows developers to select the optimal polymer for their therapeutic, whether the goal is durable immune protection, tunable release, or mechanical resilience.
How are the products administered?
- Administration routes: CSS® microspheres are typically administered via injection through a needle or infused through a small catheter, depending on the target site and dose. In addition, topical versions of the product have been developed.
- Needle size: The needles used for injection are those typically used for slightly viscous drugs like hormones and steroids.
- Injection routes: There are few limitations on where the products can be placed in the body. One exception is IV, the microbeads are too large to be administered into the vascular system.
All are compatible with standard syringe and catheter delivery systems.
How do you validate biocompatibility?
- Histological analysis: tissue response vs untreated controls (e.g., immune rejection vs CSS®-encapsulated delivery, as shown in Likarda data).
- In vitro assays: cytotoxicity
- In vivo studies: foreign body response (fibrosis, inflammation) and functional outcomes in relevant animal models.
- Materials dossier: starting materials are selected are GRAS-listed or clinically precedented polymers, reducing regulatory burden.
What’s the IP & licensing model?
- Intellectual Property: Likarda’s technology is protected by a global patent estate (50+ issued/pending patents in 20+ countries) covering the process, formulations, and applications across cells, biologics, and small molecules.
- Licensing model:
- Field-of-use or indication-specific licensing (exclusive or non-exclusive).
- Stage-gated terms: feasibility → development → commercialization.
- Economics: upfront fees + milestone payments, and possible royalties. Flexible and Negotiable.
- Equipment: The EncapSure™ instrument is purchased separately by CDMOs/partners and is not part of the licensing package.
CSS® Applications
CSS® Feasibility Studies
Quickly assess the compatibility of CSS® with your therapeutic with a low-lift feasibility program. Tailored formulations, bead sizes, and release kinetics evaluated in weeks, not months.