Introduction
Biologics have emerged as the cornerstone of modern medicine, revolutionizing the treatment of numerous diseases and conditions. These complex molecules, derived from living organisms, offer unprecedented specificity and efficacy in targeting various pathological processes. From monoclonal antibodies used in cancer therapy to recombinant proteins treating genetic disorders, biologics have dramatically improved patient outcomes and quality of life. Their ability to interact with specific cellular targets with high precision has made them indispensable in the treatment of chronic conditions, autoimmune disorders, and previously untreatable diseases.
Despite their remarkable therapeutic potential, biologics face significant challenges in the areas of formulation and delivery. Their large molecular size, complex structure, and sensitivity to environmental factors often lead to stability issues, reduced bioavailability, and the need for frequent administration. These limitations have spurred the search for innovative formulation strategies to enhance the efficacy and usability of biologic drugs.
Enter hydrogels: a versatile class of materials that have shown remarkable promise as a formulation strategy for biologics. These three-dimensional networks of hydrophilic polymers can absorb vast amounts of water while maintaining their structure, offering a unique environment for the incorporation and controlled release of biologics. Hydrogels can be tailored to provide protection against degradation, control release kinetics, and even respond to specific physiological stimuli, making them ideal candidates for biologic drug delivery.
Benefits and Challenges of Hydrogel Formulations for Biologics
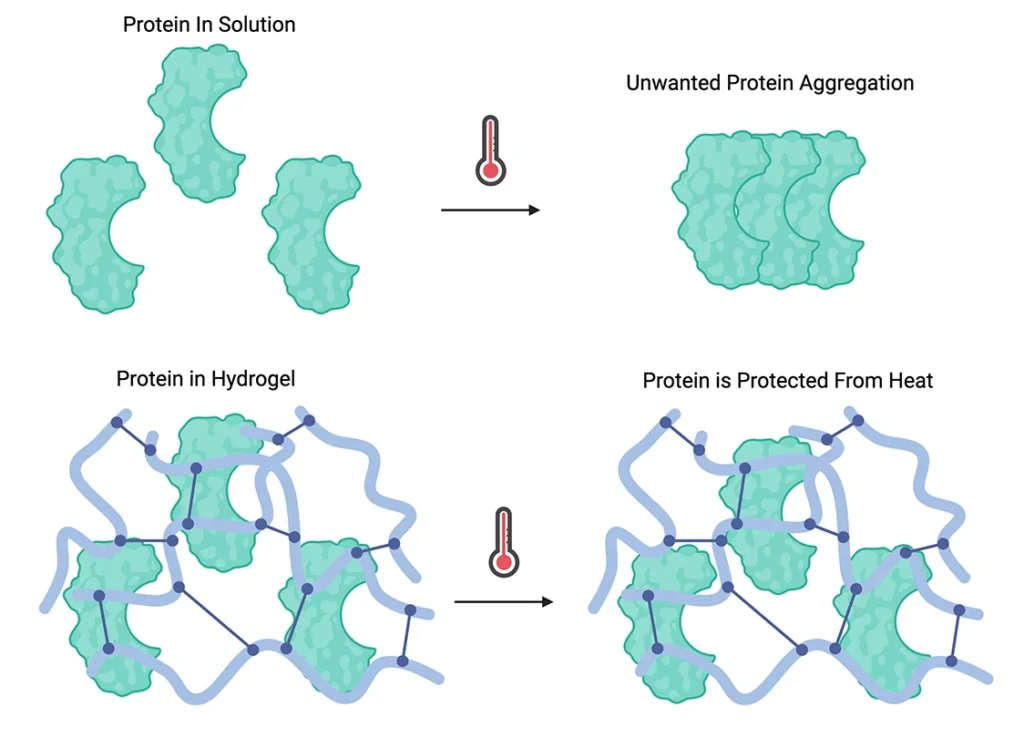
The rationale for using hydrogels with biologics stems from their capacity to protect these sensitive molecules from degradation, control release kinetics, and maintain bioactivity. Biologics can be incorporated into hydrogels through various methods, including physical entrapment, covalent binding, or affinity-based interactions. Hydrogels also have the option to be lyophilized and rehydrated later to help with biologic stability. The choice of incorporation method depends on the specific biologic and desired release profile. Release mechanisms from hydrogels typically involve diffusion, hydrogel degradation, or a combination of both. The release kinetics can be tailored by adjusting hydrogel properties such as polymer chosen, crosslinking density, mesh size, and degradation rates. This versatility allows for the design of hydrogel-biologic systems with controlled release profiles, ranging from rapid initial burst to sustained long-term delivery, enhancing the therapeutic efficacy and reducingthe frequency of administration for various biomedical applications.
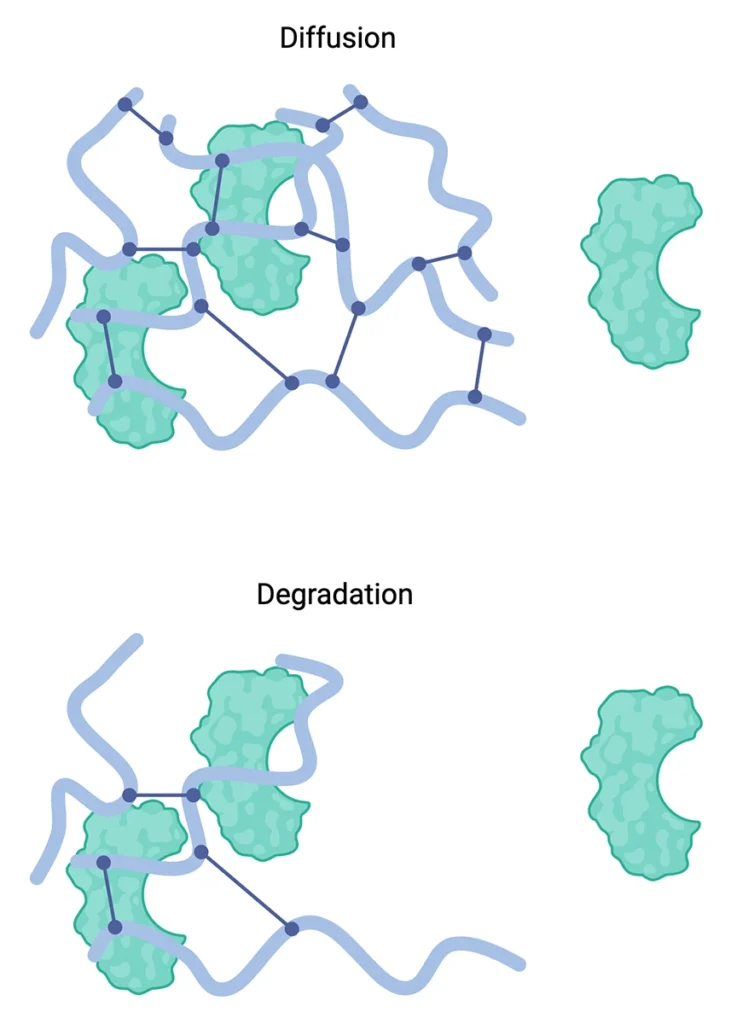 The integration of hydrogels with biologics presents significant challenges in manufacturing and scale-up. Ensuring consistent crosslinking, uniform incorporation of biologics, and maintaining sterility during production can be complex, especially when transitioning from lab-scale to commercial-scale production. This often requires substantial process optimization and investment in specialized equipment. Regulatory considerations add another layer of complexity, as authorities may demand extensive characterization of the hydrogel-biologic system, including stability studies, release profiles, and biocompatibility assessments. The novelty of some hydrogel formulations may necessitate more comprehensive safety and efficacy data for approval. Despite their advantages, hydrogel-biologic systems face potential limitations such as limited loading capacity for some biologics, potential immunogenicity of certain hydrogel materials, challenges in achieving precise control over release kinetics, possible alterations in the biological activity of encapsulated molecules, and cost considerations for novel or specialized formulations. Addressing these issues requires careful material selection, formulation optimization, and thorough preclinical evaluation to ensure the benefits outweigh any drawbacks for specific applications.
The integration of hydrogels with biologics presents significant challenges in manufacturing and scale-up. Ensuring consistent crosslinking, uniform incorporation of biologics, and maintaining sterility during production can be complex, especially when transitioning from lab-scale to commercial-scale production. This often requires substantial process optimization and investment in specialized equipment. Regulatory considerations add another layer of complexity, as authorities may demand extensive characterization of the hydrogel-biologic system, including stability studies, release profiles, and biocompatibility assessments. The novelty of some hydrogel formulations may necessitate more comprehensive safety and efficacy data for approval. Despite their advantages, hydrogel-biologic systems face potential limitations such as limited loading capacity for some biologics, potential immunogenicity of certain hydrogel materials, challenges in achieving precise control over release kinetics, possible alterations in the biological activity of encapsulated molecules, and cost considerations for novel or specialized formulations. Addressing these issues requires careful material selection, formulation optimization, and thorough preclinical evaluation to ensure the benefits outweigh any drawbacks for specific applications.
Case Studies & Future Directions
There are just over 30 injectable hydrogel-based products on the market, of those about 4 contain a biologic. Vantas and Supprellin LA are both histrelin acetate combined with poly(2-hydroxyethyl methacrylate). INFUSE® Bone Graft and OP-1 are both collagen with bone growth factor proteins. However, there are several hydrogels formulations that contain biologics in clinical trials and in discovery programs. Merck in collaboration with MIT are working on hydrogels for pembrolizumab (Keytruda) to help overcome their issues with concentrated formulations of pembrolizumab. At higher concentrations pembrolizumab becomes unstable and loses its efficacy. In another example, Ocular Therapeutix developed a sustained-release drug therapy using a biocompatible and bioresorbable hydrogel matrix that encapsulates proteins to provide sustained and localized delivery in the eye. At Likarda, with NovoSphere™, we aim to meticulously craft our hydrogels to meet your biologic drug delivery needs and formulate them into compact spherical beads that can be administer subcutaneously. All of our hydrogel precursors are biocompatible and biodegradable, easing regulatory concerns.
Summary
In conclusion, the integration of hydrogels with biologics represents a promising frontier in drug delivery, offering a sophisticated approach to overcoming the challenges associated with biologic therapies. By providing protection against degradation, controlling release kinetics, and maintaining bioactivity, hydrogels have the potential to significantly enhance the efficacy and usability of biologic drugs. However, the path to widespread adoption is not without its hurdles. Manufacturing complexities, regulatory challenges, and potential limitations in formulation require careful consideration and innovation. As ongoing research and development continue to refine these systems, the future of hydrogel-biologic formulations looks bright, with the potential to transform the landscape of modern medicine.