Macrodevices for Cell Therapies
When discussing cell therapies, the term macroencapsulation indicates a device that is larger than 1 mm in dimensions that is designed to protect cells from an immune system attack.
In Part I of this series, we reviewed the advantages and disadvantages of the general category of macrodevices as a delivery system. In this installation, the history of macrodevice design and current trends in the field will be discussed. The discussion is organized according to different categories of cell therapy macrodevices. Each will be reviewed with an emphasis on the results of human clinical trials when applicable.
Macrodevice Categories
There are five general categories of macrodevices we will examine in this article. These include:
- Hollow fibers
- Hydrogel patches
- Bilaminar (wafer) devices
- Replenishable reservoirs
- Microneedle patches
Hollow Fibers
Hollow fiber devices were some of the first approaches studied starting in the early 1990s. They have been manufactured from a number of different materials1. The concept is simple, maximize the surface area of the device while minimizing the distance of any cell to the surface. With the optimal surface-to-volume ratio being small, a long thin fiber shape appeared to be the best option for a macrodevice. The fibers can be manufactured from a number of biocompatible materials and typically have an alginate core to hold the cells in place.
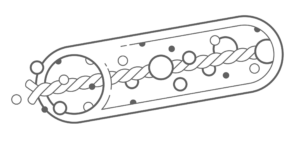
While the majority of these devices are hollow, some designs include a suture thread or suture-like material at the center with a hydrogel, often alginate, attached to the surface as shown in Figure 12,3.
Early on, fiber-based devices were focused on the treatment of neurological diseases. For example, in treating Huntington’s disease, genetically modified cells were placed in hollow poly(acrylonitrile-co-vinyl chloride) fibers filled with a collagen matrix. The human clinical trial for this approach was initiated in 2004 with no published results4. Vision loss has been targeted by inserting human retinal pigment epithelium cells into a polysulfone hollow-fiber for the release of neurotrophins5. Cells to treat Parkinson’s disease, epilepsy and Alzheimer’s disease have all been in preclinical or clinical trials using hollow fibers5. In each case, a relatively small volume of cells were transplanted.
Later versions of the fiber approach were taken up at Ma’s lab at Cornell. They fabricated electrospun fiber scaffolds with a silicone-polycarbonate-urethane for treatment of diabetes. The cells were mixed in an alginate solution and injected into the center of the fibers, then implanted subcutaneously. Thus far publications describing the approach have only achieved normal blood glucose levels for 60 days in immunocompetent mice6.
With chronic diseases requiring high numbers of transplanted cells such as in the treatment of diabetes, the hollow fiber approach has its limitations. The constraints of the fiber diameter can induce core cell necrosis due to the diffusion barrier7. The challenge of adequate diffusion of nutrients and waste molecules in and out of the fiber is a consistent issue for macrodevices, as discussed in Part I . More importantly, the small volume of the fiber format limits the number of cells that can be packed into a given area. This translates to extremely long fibers to treat a systemic disease like diabetes. For example, some have estimated that an adult with type 1 diabetes would require anywhere from a 17 meter long hollow fiber, with some estimates of 70 meters in length required to reverse diabetes1,8. Obviously, these calculations show that the simple hollow tube format has important limitations for certain applications with high cellular requirements.
Hydrogel Patches
As the realities of hollow tube limitations came into view in the 1990s, researchers turned to patches of hydrogel that could sustain cells long term. The term patch indicates that the device is comprised of a homogenous sponge-like hydrogel as shown in Figure 2.
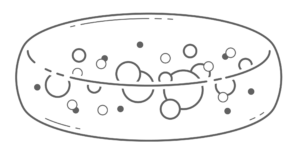
Patches may be assembled onto more complex chips or needle devices, discussed below. Alginate was the firsthydrogel used in this fashion due to its crosslinking properties making it easy to produce9.
Cardiac diseases have been the focus of many of the patch designs. Simple designs comprised of a PVA stamp encapsulating multipotent stromal cells (MSCs) resulted in improved cardiac remodeling in a rat myocardial infarction model10. Later, improved cardiac function was measured in mice after myocardial infarction with the insertion of a cardiac patch loaded with anti-oxidative molecules together with MSCs.
More complex fabrication of patch macrodevices started with an aldehyde-dextran sponge loaded with the molecules and bonded to the heart. Once attached, the device is filled with an HA solution containing MSCs11. In rats, they found that both the ejection fraction and the fractional shortening of the heart were improved over the control group.
Several improvements have been made to the basic hydrogel patch, most importantly moving away from alginate, which induces a strong foreign body response by the host. For example, a patch comprised of biocompatible molecules such as collagen and hyaluronic acid has been used to encapsulate insulin-producing islet cells and transplant them into diabetic rats, which reversed diabetes for 18 months without immunosuppression12. Tiang et al developed a PVA patch that was prepared with the cells. The patch was attached to an apparatus that included multiple cone-shaped needles that allowed the patch to stay on the surface of the heart even through the normal movement associated with heart contractions13. In a pig model of heart disease, the cells halted a decline in cardiac function. Similar needle arrays have been used in the treatment of type 1 diabetes. In this example, cell-containing alginate microparticles were attached to a hyaluronic acid needle array and applied transcutaneously14. The researchers showed normalization of blood glucose in diabetic mice for several hours.
Recently cell delivery patches have been directed at solid tumors as carriers for CAR-T cells. One iteration was developed from a thin film of nitrinol. In mouse studies the patches were able to eradicate tumors 70% of the time with an extended average survival time for the mice15. The advantage of such applications is that the device can also be impregnated with factors that maintain the health of the T-cells and enhance proliferation and function16,17.
Bilaminar Devices
Bilaminar devices typically start out with an internal patch construct that is covered with another substance resulting in two membranes with a depot in the core where cells are implanted as shown in Figure 3.
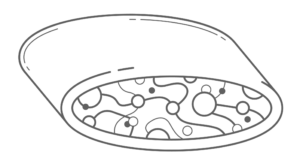
The design provides an advantage, because the core materials can be chosen to optimize the environment for the transplanted cells, where the material used for the surface can be chosen to have good biocompatibility properties17. While these devices may have excellent immune protection for the entrapped cells and be easily retrieved, diffusion into and out of the device has been a reoccurring issue predominantly because multiple layers of material are on the surface, which impedes diffusion of nutrients to the cells18.
Early on, the company ViaCyte led the way in developing a bilaminar device called PEC-01. While the device was found to be safe, it instigated a strong immune rejection response that resulted in failure of the graft. Later versions included large pores into the core of the device allowing vessels to grow into the compartment with the cells as a way to reduce the diffusion barrier. However, as blood vessels grew into the device, the immune protection was negated6. The introduction of expanded polytetrafluoroethylene (ePTFE) improved the biolayer encapsulation approach as the material is both immune protective and angiogenic6. TheraCyte has a similar device with an outer layer of woven polyester mesh and inner layers of PTFE19. Unfortunately the device has shown significant activation of a foreign body response surrounding the implant that negates the activity of the cells20. Still PTFE and PCTE based bilaminar devices continue to be popular.
Recognizing the poor diffusivity of the macrodevice category, the company Beta O2 was one of the first to devise a cell device that added oxygen to the core to prevent cell death. Their device also utilizes ePTFE as the outer shell, which was filled with cells in alginate. The core of the device is connected to two ports that supply external oxygen to the trapped cells17. This approach overcomes the diffusion barrier found in other devices but increases the risk of infection21. Implantation into a single diabetic patient demonstrated functional cells even at 9 months with the production of C-peptide and improved hemoglobin A1C levels. However, exogenous insulin was still required22. The next generation of such a device was developed by Procyon Technologies with the inclusion of an oxygen generator that is currently designed to be worn on the wrist, with plans to eventually create an oxygen generator small enough to be implanted6. Others have also created a transcutaneous device that is in direct contact with the atmosphere23. With all of these macrodevices, the risk of infection or trauma to the site of a superficial implant is significant.
Replenishable Reservoirs
The term “replenishable reservoirs” describes devices in which the cells can be added after implantation and potentially replenished in the device, if they fail at any point. One of the first replenishable devices was developed in Alberta Canada24. The device was placed under the skin of diabetic mice and after the vasculature had grown into the device, it was loaded with syngeneic islets. The results showed a return to normoglycemia in the mice without immune suppression24. Later perturbations of the device contained a set of small parallel tubes running through the center where cells were embedded. In the first clinical trial targeting people with type 1 diabetes, there was an initial increase in C-peptide release, but it was not sustained25. While diffusion limits have been overcome with this approach, because blood vessels grow into the device, the immunosuppression effects of the device are often lost. A device from this category is being used by Sernova in clinical trials and was shown to improve blood glucose regulation, but it alone did not reverse hyperglycemia in humans26.
Beyond diabetes, such appliances have been used to target neurological diseases. Scientists from Hoffmann-La Roche implanted genetically engineered cells that secreted high levels of anti-amyloid-β antibodies into mice. The device was placed under the skin allowing blood vessels to grow into the device, and then the device was filled with cells through a port. They showed a reduction in amyloid-β levels in the brain and a decrease in the amyloid plaque burden in the Alzheimer’s mouse model27.
More complex multicompartment models have been developed. One iteration called Neovascularized Implantable Cell Homing and Encapsulation (NICHE), was used to protect testosterone-producing Leydig cells from destruction by the rat’s immune system28. Adjacent to the cell chamber were two drug reservoirs filled with immunosuppressing drugs. The system demonstrated sustained release of the immunosuppressant and functional Leydig cells in rats.
While the replenishable devices offer a great advantage in the long-term correction of a systemic disease that would require life-long treatment, they continue to have issues with diffusion and most lack the ability to protect cells from the immune system. Thus, the recipient must take systemic immune-suppressing drugs for the lifetime of the transplant.
Conclusion
The macrodevice field is a rapidly developing and expanding area of research with new device designs presented monthly and iterations of older devices being tested clinically. The macrodevice approach will clearly be an advantage for treatment of specific diseases, such as heart injury when a patch attached to the myocardium holds the cells to the surface of the beating heart long enough to have an impact. Conversely, using a macrodevice design to treat diseases that require a large number of cells to be maintained long term is less likely for the following reasons:
- Significant diffusion barrier limits the longevity of the trapped cells.
- The large cell numbers needed would result in high numbers of devices or meters of fiber which would be difficult to insert correctly.
- Many devices designed to overcome the diffusion barrier, lose their ability to protect the cells from immune attack.
For these reasons, it may be best to consider macrodevices for the delivery of cells for short term requirements, such as mending the myocardium after a heart attack, to delivering anti-cancer cells or to regenerate an attachment between severed ends of a nerve. However, for long-term therapeutic applications such as treating hypothyroidism or diabetes, the macrodevice approach may not be as beneficial.
Have a Delivery System Issue?
If you believe you have a delivery system issue with a cell therapy currently in development, or you’re planning on developing a new cell therapy and want to avoid delivery system issues, learn more about how Likarda’s targeted delivery system can help you.
Or contact us today.
Resources
1. C. Colton, Oxygen supply to encapsulated therapeutic cells, Adv Drug Deliv Rev 67-68 (2014) 93-110.
2. R. Costa-Almeida, I. Calejo, R. Altieri, R. Domingues, E. Giordano, R. Reis, M. Gomes, Exploring platelet lysate hydrogel-coated suture threads as biofunctional composite living fibers for cell delivery in tissue repair, Biomed Mater 14(3) (2019) 034104.
3. P. Lauren, P. Somersalo, I. Pitkanen, Y.-R. Lou, A. Urtti, J. Partanen, J. Seppala, M. Madetolja, T. Laarksonen, A. Makitie, M. Yliperttula, Nanofibrillar cellulose-alginate hydrogel coated surgical sutures as cell-carrier systems, PLoS One 12(8) (2017) e0183487.
4. J. Bloch, A. Bachoud-Levi, N. Deglon, J. Lefaucheur, L. Winkel, S. Palfi, J. Nguyen, C. Bourdet, V. Gaura, P. Remy, P. Brugieres, M. Boisse, S. Baudic, P. Cesaro, P. Hantraye, P. Aebischer, M. Peschanski, Neuroprotective gene therapy for Huntington’s disease, using polymer-encapsulated cells engineered to secrete human ciliary neurotrophic factor: results of a phase I Hum Gene Ther 15 (2004) 968-975.
5. M. Zanin, L. Pettingill, A. Harvey, D. Emerich, C. Thanos, R. Shepherd, The development of encapsulated cell technologies as therapies for neurological and sensory diseases, J Control Release 160(1) (2012) 3-13.
6. H. Dang, H. Chen, T. Dargavelle, B. Tuch, Cell delivery systems: toward the next generation of cell therapies for type 1 diabetes, J Cell Mol Med 26 (2022) 4756-4767.
7. D. Scharp, P. Marchetti, Encapsulated islets for diabetes therapy: history, current progress, and critical issues requiring solution, Adv Drug Deliv Rev 67-68 (2014) 35-73.
8. G. Erdodi, J. Kang, B. Yalcin, M. Cakmak, K. Rosenthal, A novel macroencapsulating immunoisolatory device: the preparation and properties of nanomat-reinforced amphiphilic co-networks deposited on perforated metal scaffold, Biomed Macrodevices 11 (2009) 297-312.
9. R. Storrs, R. Dorian, S. King, J. Lakey, H. Rilo, Preclinical development of the islet sheet, Ann NY Acad Sci 944 (2001) 252-266.
10. I. Kim, M. Hwang, J. Park, S.-H. Kim, J.-H. Kim, H. Kang, R. Subbiah, U. Ko, J. Shin, C.-H. Kim, D. Choi, K. Park, Stretchable ECM patch enhances stem cell delivery for post-MI cardiovascular repair, Adv Healthcare Mat 8(17) (2019) 1900593.
11. T. Wu, X. Zhang, Y. Liu, C. Cui, Y. Sun, W. Liu, Wet adhesive hydrogel cardiac patch loaded with anti-oxidative, autophagy-regulating molecule capsules and MSCs for restoring infarcted myocardium, Bioact Mater 21(7) (2023) 029.
12. S. Harrington, S. Williams, S. Rawal, K. Ramachandran, L. Stehno-Bittel, Hyaluronic acid/collagen hydrogel as an alternative to alginate for long-term immunoprotected islet transplantation, Tissue Eng Part A 23(19-20) (2017) 1088-1099.
13. J. Tang, K. Huang, Y. Ye, T. Su, L. Qiao, M. Hensley, T. Caranasos, J. Zhang, Z. Gu, K. Cheng, Cardiac cell-integrated micro needle patch for treating myocardial infarction, Sci Adv 4(11) (2018) eaat9365.
14. Y. Ye, J. Yu, C. Wang, N.-Y. Nguyen, G. Walker, J. Buse, Z. Gu, Microneedles integrated with pancreatic cells and synthetic glucose-signal amplifiers for smart insulin delivery, Adv Mater 28(16) (2016) 3115-3121.
15. M. Coon, S. Stephan, V. Gupta, C. Kealey, M. Stephan, Nitrinol thin films functionalized with CAR-T cells for the treatment of solid tumors, Nat Biomed Engineer 4(2) (2020) 195-206.
16. Q. Hu, H. Li, E. Archibong, Q. Chen, H. Ruan, S. Ahn, E. Dukhovlinova, Y. Kang, D. Wen, G. Dotti, Z. Gu, Inhibition of post-surgery tumor recurrence via a hydrogel releasing CAR-T cells and anti-PDL1-conjugated platelets, Biomed Eng 5 (2021) 1038-1047.
17. W. Liu, Y. Wang, J. Wang, O. Lanier, M. Wechsler, N. Peppas, Z. Gu, Macroencapsulation devices for cell therapy, Engineering 13 (2022) 53-70.
18. R. Chang, G. Faleo, H. Russ, A. Parent, S. Elledge, D. Bernards, J. Allen, K. Villanueva, M. Hebrok, Q. Tang, T. Desai, Nanoporous immunoprotective devices for stem cell derived b-cell replacement therapy, ACS Nano 11(8) (2017) 7747-7757.
19. R. Geller, T. Loudovaris, S. Neuenfeldt, R. Johnson, J. Brauker, Use of an immunoisolation device for cell transplantation and tumor immunotherapy, Ann NY Acad Sci 831(1) (1997) 438-451.
20. A. Tibell, E. Rafeal, L. Wennberg, J. Nordenstrom, M. Bergstrom, R. Geller, T. Loudovaris, R. Johnson, J. Brauker, S. Neuenfeldt, A. Wernerson, Survival of macroencapsulated allogeneic parathyroid tissue one year after transplantation in nonimmunosuppressed humans, Cell Transplantation 10(7) (2001) 591-599.
21. B. Ludwig, B. Zimerman, A. Steffen, K. Yavriants, D. Azarov, A. Reichel, P. Vardi, T. German, N. Shabtay, A. Rotem, Y. Avron, T. Neufeld, S. Mimin, S. Ludwig, M. Brendel, S. Bornstein, U. Barkai, A novel device for islet transplantation providing immune protection and oxygen supply, Horm Metab Res 42(13) (2010) 918-922.
22. B. Ludwig, A. Reichel, A. Steffen, B. Zimmermann, A. Schally, N. Block, C. Colton, S. Ludwig, S. Kersting, E. Bonifacio, M. Solimena, Z. Gendler, A. Rotem, U. Barkai, S. Bornstein, Transplantation of human islets without immunosuppression, PNAS 110(47) (2013) 19054-19058.
23. D. An, L.-H. Wang, A. Ulrich, A. Chiu, Y.-C. Lu, J. Flanders, A. Datta, M. Ma, An atmosphere-breathing refillable biphasic device for cell replacement therapy, Adv Mater 31(52) (2019) e1905135.
24. A. Pepper, R. Pawlick, B. Gala-Lopez, A. MacGillivary, D. Mazzuca, D. White, P. Toleikis, A. Shapiro, Diabetes is reversed in a murine model by marginal mass syngeneic islet transplantation using a subcutaneous cell pouch device, Transplantation 99(11) (2015) 2294-2300.
25. B. Gala-Lopez, A. Pepper, P. Dinyari, A. Malcolm, T. Kin, L. Pawlick, P. Senior, A. Shapiro, Subcutaneous clinical islet transplantation in prevascularized subcutaneous pouch – preliminary experience, CellR4 4(5) (2016) e2132.
26. P. Bachul, G. Generette, A. Perez-Gutierrez, P. Borek, L.-J. Wang, K. Golab, L. Basto, L. Perea, M. Tibudan, B. Juengel, J. Kumar, C. Thomas, L. Philipson, J. Fung, P. Witkowski, Modified approach allowed for improved islet allotransplantation into pre-vascularized Sernova Cell Pouch Device – Preliminary results of the Phase I/II clinical trial at University of Chicago, Transplant 105(1241) (2021) pS25.
27. A. Lauthuiliere, V. Laversenne, A. Astolfo, E. Kopetzki, H. Jacobsen, M. Stampanoni, B. Bohrmann, B. Schneider, P. Aebischer, A subcutaneous cellular implant for passive immunization against amaloid-b reduces brain amyloid and tau pathologies, Brain 139 (2016) 1587-1604.
28. J. Paez-Mayorga, S. Capuani, N. Hernandez, M. Farina, C. Chua, R. Blanchard, A. Sizovs, H.-C. Liu, D. Fraga, J. Niles, H. Slazar, B. Corradetti, A. Sikora, M. Kloc, X. Li, A. Gaber, J. Nichols, A. Grattoni, Neovascularized implantable cell homing encapsulation platform with tunable local immunosuppressant delivery for allogeneic cell transplantation, Biomat 257 (2020) 120232.
