Introduction
In the recent past, the field of regenerative medicine has gained increasing prominence as a new category of therapies with the ability to cure chronic diseases. Continued innovations in deriving new sources and classifications of stem cells have propelled the field forward. Stem cells can be thought of as the starting material for a cell therapy and they hold immense potential to treat diseases and conditions that otherwise have limited or no treatment options.
Stem cells are simply the master template from which all organs and tissues in the body develop. Thus, during the formation and development of an animal or human a continuous source of new cells is needed to replace dying cells. This feat is achieved by stem cells, which have two major defining characteristics that allow for their success:
- i) Self-renewal in order to maintain their own continuous supply
- ii) Differentiation to generate new cell types and tissues in the body
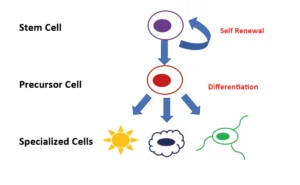 These two features of stem cells are shown in the figure illustrating the pathways for self-renewal or differentiation into precursor cells that can further be differentiated into mature, specialized cells.
These two features of stem cells are shown in the figure illustrating the pathways for self-renewal or differentiation into precursor cells that can further be differentiated into mature, specialized cells.
All stem cells are not created equal, but rather exist in a developmental hierarchy, which is defined by their source, and/or their capacity to be differentiated into different cell types. From the early stages of a fertilized egg into adulthood, stem cells with different characteristics and differentiation capacities have been isolated and characterized.
Classification of Stem Cells
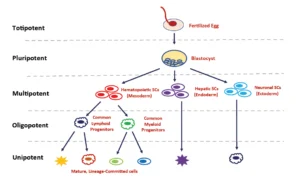 Based on their potential to mature into unique tissues, stem cells can be classified into one of five categories as shown in the figure. The schematic illustrates the stem cell hierarchy based on the development and differentiation potential. At the apex is the Totipotent Stem Cell, which has capacity to produce all of the cells of the entire human body. From there, the ability to mature into different kinds of tissues decreases all the way down the chain to Unipotent Cells that are capable of dividing but cannot be differentiated into different types of cells or tissues.
Based on their potential to mature into unique tissues, stem cells can be classified into one of five categories as shown in the figure. The schematic illustrates the stem cell hierarchy based on the development and differentiation potential. At the apex is the Totipotent Stem Cell, which has capacity to produce all of the cells of the entire human body. From there, the ability to mature into different kinds of tissues decreases all the way down the chain to Unipotent Cells that are capable of dividing but cannot be differentiated into different types of cells or tissues.
i) Totipotent stem cells can differentiate into cells that can create the whole organism. Totipotent cells are at the top of the hierarchy tree. An example would be a fertilized egg.
ii) Pluripotent stem cells can give rise to all tissues except the extraembryonic tissues such as the placenta. In humans, pluripotent stem cells can be isolated from 4-day old fertilized eggs and are termed Embryonic Stem Cells (ESCs), which require special precautions for their isolation and growth. More recently, scientists have been able to reprogram adult Unipotent Cells such as fat cells into pluripotent cells by using defined factors and gene editing to derive what is termed Induced Pluripotent Stem Cells (iPSCs).
iii) Multipotent stem cells are more restricted to make certain kinds of cells of specific lineages. One example is the hematopoietic stem cell, which can differentiate into all cells of the blood including red blood cells and various types of white blood cells, but these cells do not normally differentiate into non-blood cells such as tissues or organs.
iv) Oligopotent stem cells are even more restricted in their capacity to convert into different types of cells. An example would be the myeloid stem cells that are capable of making only white blood cells but not red blood cells.
v) Unipotent stem cells are only able to form one cell type and are extremely limited in their potential, although they are capable of dividing repeatedly. Thus, a muscle cell, for example, can divide and make new muscle cells, but could not differentiate into a liver cell.
Sources of Stem Cells
Stem cells can be isolated from many varied sources and can be used in creating cells therapies. Those stem cell sources fall into one of two major categories, namely:
i) Pluripotent Stem Cells
ii) Adult Stem Cells.
Pluripotent stem cells are best exemplified by ESCs, which in humans are derived from developing blastocysts. Due to the ethical concerns surrounding the source and derivation of ESCs from developing blastocysts, they have lost favor with most researchers. Investigators have shifted their research to using the less controversial, iPSCs. iPSCs are derived from adult cells that are re-programmed back into a more primitive or undifferentiated state. Thus, no blastocysts are required for their production.
Adult stem cells are generally found in small numbers in most adult tissues including bone marrow, fat, skin and muscle, brain, etc. They are also isolated from perinatal tissues such as amniotic fluid and umbilical cord blood.
Why are Stem Cells of So Much Interest?
Stem cells have continued to generate a lot of interest in the research community.
1) Research in Growth and Developmental Models
In studying how stem cells develop and organize into various tissues and organs of the body, scientists hope to learn more on the biological processes and pathways of maturation, allowing a better understanding of healthy development and how diseases arise and progress.
2) Drug Screening
Investigational new drugs require significant testing for safety and quality including toxicological analysis before they are used in humans. Stem cells and tissues organoids similar to the target tissue in the body are used to test the new drugs and provide a more predictive response to the drugs.
3) Regenerative Medicine
In addition to research, stem cells can be used to generate new healthy cells that can replace damaged or diseased tissues and organs of the body. This field is called Regenerative Medicine and is being used to target diseases including Type 1 diabetes, Parkinson’s disease, heart disease, stroke, burns and cancers. Stem cells and their derivatives act to i) replace damaged tissue ii) help regenerate the patient’s own tissues or iii) promote the recruitment of other cells to the damaged or inflamed sites, which then secrete beneficial factors to promote the healing process.
Challenges of Using Stem Cells to Treat Disease
Despite all the progress and promise offered by stem cells to cure many diseases in human and veterinary medicine, there are still major obstacles and challenges to be overcome before the widespread administration of stem cells to address chronic diseases can be realized. They include:
1) Differentiation protocols: Robust, reproducible, and directed differentiation protocols are needed for various diseases such as Type 1 diabetes and Parkinson disease. It is not enough to simply inject undifferentiated stem cells into a patient and hope that they know what to do. Rather, the cells are typically first differentiated down one of the pathways shown in the second figure. Currently, different groups are using custom protocols for differentiating the stem cells into biologically functional or adult-like tissues, but often these protocols are not reliably reproducible, and do not always translate from source of cells to another.
2) Immune rejection: When the therapeutic stem cells or their progenies are sourced from a donor and placed in the patient’s or recipient’s body, they often suffer attack from the host’s immune system. This remains one of the greatest challenges hindering the use of stem cells for therapeutic purposes. Efforts to mitigate immune rejection include the use of immunosuppressive drugs, and strategies to encapsulate or coat the cells so that they will not be detected by the immune system. Alternative efforts include genetic engineering to produce “immunologically silent” adult cells from stem cells by using CRISPR technology or other genetic approaches.
3) Tumor formation: The hallmark feature of a stem cell in the growing blastocyst is to divide and produce new cells of different kinds. The threat of uncontrolled cell division can result in unwanted cancer cells. To mitigate this challenge, some approaches being used include: (a) sorting of the cells to remove any remaining pluripotent cells in the final therapy product, (b) continuous improvements of differentiation methods to minimize or reduce the levels of undifferentiated cells to undetectable levels (c) use of suicide genes that target undifferentiated stem cells and kill them.
4) Localization of the cells: The inability to deliver and retain cell therapies into the right location in the body to achieve their desired effect has become a clear limitation for the field. A good example is when treating osteoarthritis with stem cells injected into the knee, where they are expected to remain and function locally. It has been shown that as soon as the person stands up bearing weight on the joint, most of the cells are pushed out of the joint space, thereby rendering them ineffective. Various methods of cell delivery including encapsulation and local delivery are under investigation.
5) Unknown mechanism of action: Stem cells have complex mechanisms of action and may function in more than one way. By contrast, a traditional chemical drug acts by binding to a specific receptor on a specific cell and the outcome of that binding is well understood. In contrast, cells can secrete a multitude of factors, which can change once placed in the body. Thus, predicting what the therapy will do after placement in the body can be difficult, if not impossible.
6) Regulatory concerns: Stem cells therapies are highly regulated and controlled by government agencies such as the FDA. The FDA has categorized several critical quality control attributes that any stem cell therapy must demonstrate in order for the therapy to be allowed in human clinical trials. These attributes fall into the following categories:
a) Safety – Cell therapy products must be safe from all potential harm to the patient including the ability to cause tumors or develop into other undesirable tissues or cells. Safety analysis also includes the absence of any adventitious agents such as viral, bacterial, or other microbial pathogens.
b) Identity – Cell therapy products must be fully characterized to identify not only the necessary cells needed for the treatment but also determine undesirable cell contaminants.
c) Purity – Cell therapy products need to meet high purity standards and be free from all residual contaminants either from the manufacturing processes or the original cell source.
d) Potency – Cell therapy products must meet potency standards and be medically effective for the products to be allowed into the clinic. In other words, they must do what they claim.
7) Cost: Manufacturing a cell therapy product is expensive, which is a great limitation to widespread adoption. Use of expensive starting materials, complicated manufacturing processes, complex cold-chain shipping and storage requirements, and unique administration procedures add immense cost to the final product.
8) Ethical concerns: Ethical concerns around the use and application of stem cells have been a limiting factor to widespread adoption. Methods to use reprogramming to derive iPSCs rather than using embryonic stem cells provides alternative ways and may aid in helping more widespread adoption. However, strategies need to be undertaken to better educate those in the healthcare system and the public about iPSCs and their sourcing.
9) Others: There are many other concerns and challenges that will continue to be addressed in the future for stem cell therapies to be widely accessible. These include the longevity or duration of the therapies, the possible need for repeated treatments, coverage by insurance companies, and availability in less developed economies due to cost and healthcare infrastructure.
Summary
In summary, the advances in the use of stem cells in medicine has occurred at a rapid pace. A recent search of the NIH’s repository of human clinical trials resulted in nearly 8000 studies listing stem cells as part of the treatment. The focus of these studies was varied and contained treatments for encephalopathy, Duchenne’s muscular dystrophy, neuropathies, autism, obstructive pulmonary disease, and many others. Beyond the many clinical trials underway, the FDA has already approved nearly 80 diseases to be treated with cord blood stem cells including leukemias, lymphomas, anemias, sickle cell disease, thombocytopenia, apraxia, multiple myeoloma, Hunter syndrome, neuroblastoma, and many others. The list of approved therapies is surely set to increase exponentially with so many treatments currently in human clinical trials. Thus, the burden is on each person in the healthcare industry and all consumers of healthcare to understand the basic characteristics of stem cells and their potential as well as limitations.
